文献:Chowdhury R et al.Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis.BMJ 2012;345:e6698.
魚の摂取および長鎖オメガ3脂肪酸と脳血管疾患リスクの関連を、26のコホート研究と12の無作為化比較試験のシステマティックレビューおよびメタ解析で検討。魚摂取量が1食分/週と比べ2-4食分/週の相対リスクは0.94だった。対照群と比べ長鎖オメガ3サプリメントの相対リスクは1次予防試験0.98、2次予防試験1.17だった。
Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis
BMJ2012;345doi: http://dx.doi.org/10.1136/bmj.e6698(Published 30 October 2012)
Cite this as:BMJ2012;345:e6698
Rajiv Chowdhury, research associate1, Sarah Stevens, public health registrar5, Donal Gorman, PhD candidate1, An Pan, research associate2, Samantha Warnakula, PhD candidate1, Susmita Chowdhury, research associate6, Heather Ward, research fellow4, Laura Johnson, epidemiologist1, Francesca Crowe, nutritional epidemiologist3, Frank B Hu, professor2, Oscar H Franco, professor17
Author Affiliations
1Department of Public Health and Primary Care, University of Cambridge, UK
2Department of Nutrition, Harvard School of Public Health, Boston, USA
3Cancer Epidemiology Unit, Nuffield Department of Clinical Medicine, University of Oxford, UK
4Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, UK
5Public Health Directorate, NHS Midlands and East, Fulbourn, Cambridge, UK
6Public Health Genomics Foundation, Cambridge, UK
7Department of Epidemiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands
Correspondence to: O H Franco o.franco@erasmusmc.nl
Accepted 24 September 2012
Abstract
Objective To clarify associations of fish consumption and long chain omega 3 fatty acids with risk of cerebrovascular disease for primary and secondary prevention.
Design Systematic review and meta-analysis.
Data sources Studies published before September 2012 identified through electronic searches using Medline, Embase, BIOSIS, and Science Citation Index databases.
Eligibility criteria Prospective cohort studies and randomised controlled trials reporting on associations of fish consumption and long chain omega 3 fatty acids (based on dietary self report), omega 3 fatty acids biomarkers, or supplementations with cerebrovascular disease (defined as any fatal or non-fatal ischaemic stroke, haemorrhagic stroke, cerebrovascular accident, or transient ischaemic attack). Both primary and secondary prevention studies (comprising participants with or without cardiovascular disease at baseline) were eligible.
Results 26 prospective cohort studies and 12 randomised controlled trials with aggregate data on 794 000 non-overlapping people and 34 817 cerebrovascular outcomes were included. In cohort studies comparing categories of fish intake the pooled relative risk for cerebrovascular disease for 2-4 servings a week versus ≤1 servings a week was 0.94 (95% confidence intervals 0.90 to 0.98) and for ≥5 servings a week versus 1 serving a week was 0.88 (0.81 to 0.96). The relative risk for cerebrovascular disease comparing the top thirds of baseline long chain omega 3 fatty acids with the bottom thirds for circulating biomarkers was 1.04 (0.90 to 1.20) and for dietary exposures was 0.90 (0.80 to 1.01). In the randomised controlled trials the relative risk for cerebrovascular disease in the long chain omega 3 supplement compared with the control group in primary prevention trials was 0.98 (0.89 to 1.08) and in secondary prevention trials was 1.17 (0.99 to 1.38). For fish or omega 3 fatty acids the estimates for ischaemic and haemorrhagic cerebrovascular events were broadly similar. Evidence was lacking of heterogeneity and publication bias across studies or within subgroups.
Conclusions Available observational data indicate moderate, inverse associations of fish consumption and long chain omega 3 fatty acids with cerebrovascular risk. Long chain omega 3 fatty acids measured as circulating biomarkers in observational studies or supplements in primary and secondary prevention trials were not associated with cerebrovascular disease. The beneficial effect of fish intake on cerebrovascular risk is likely to be mediated through the interplay of a wide range of nutrients abundant in fish.
Introduction
Fish consumption is considered one of the key components of a cardioprotective diet.1 Current cardiovascular guidelines2 3 for healthy individuals encourage consumption of a variety of fish, preferably oily types, at least twice a week. Cold water oily fish and fish oil are also the most common dietary sources of long chain omega 3 fatty acids, a group of polyunsaturated fats that primarily include eicosapentaenoic acid and docosahexaenoic acid.4 Studies show that regular consumption of both these fats as supplements may reduce arrhythmias, endothelial dysfunction, circulating triglyceride levels, and inflammation.5 6 Such findings have prompted clinical guidelines to recommend the use of these nutrients in people with pre-existing coronary heart disease or high blood levels of triglycerides.7 8 Whether, or to what extent, current recommendations for consumption of fish or long chain omega 3 fatty acids may apply to cerebrovascular diseases is, however, unclear. Observational evidence on consumption of fish and omega 3 fatty acid is inconsistent for cerebrovascular outcomes.9 Likewise, despite a growing body of experimental evidence on eicosapentaenoic acid and docosahexaenoic acid supplements, the results for cardiovascular prevention are conflicting. Whereas earlier trials reported moderate protective effects on coronary heart disease and sudden cardiac death outcomes,10 11 recent large scale primary and secondary prevention trials failed to show the efficacy of supplementation with long chain fatty acids in reducing cardiovascular diseases.12 13 Therefore, whether supplementing with long chain omega 3 fatty acids could specifically help prevent stroke, remains elusive.
Several studies have investigated the intake of fish and long chain omega 3 fatty acids in relation to cerebrovascular disease.14 15 16 17 18 19 20 21 However, these studies varied in methodology (for example, self reported dietary intake of omega 3 fatty acids versus circulating biomarkers versus supplements), scientific rigour (for example, sufficient power and duration of follow-up), or the ability to evaluate associations in greater detail (for example, by stroke subtypes). Furthermore, previous meta-analyses22 23 24 dealing with these hypotheses did not assess important differences in the exposure type, such as white versus fatty fish consumption, within clinically relevant subgroups (for example, average intake or stroke subtypes); and did not systematically compare observational evidence in the context of experimental data in a single investigation.
We systematically reviewed and meta-analysed available studies to quantify the associations of fish consumption with total and cause specific cerebrovascular disease in prospective cohort studies; examined associations of dietary and circulating levels of long chain omega 3 fatty acids with cerebrovascular diseases in observational studies; and evaluated the potential effects of their supplementations on future cerebrovascular events in randomised controlled trials.
Methods
We carried out an electronic search using primarily Medline, supplemented by searches of Embase, BIOSIS, and the Science Citation Index databases, for studies published before September 2012, without any language restriction (see supplementary file for details of the search strategy). Potentially eligible studies were those that had reported associations of fish (defined as fish and other seafood) or omega 3 fatty acids consumption, circulating omega 3 fatty acids, and omega 3 fatty acid supplements or dietary fish interventions with cerebrovascular disease (defined as any fatal or non-fatal ischaemic stroke, haemorrhagic stroke, cerebrovascular accident, or transient ischaemic attack). In the computer based searches we combined search terms related to the exposure (for example, fish, fatty acids, omega 3 fatty acids) and outcomes of interest (stroke, cerebrovascular disorders, etc). We considered prospective cohort studies to be eligible for inclusion if they had at least one year of follow-up and involved either general populations (participants not selected on the basis of pre-existing disease) or people at high risk of cardiovascular disease (those selected on the basis of high risk factors for cardiovascular disease or pre-existing cardiovascular disease, or both). Randomised intervention studies were eligible for inclusion if they assessed the effects of long chain omega 3 fatty acids supplements or dietary fish consumption in adults; collected “hard” cerebrovascular disease endpoints, such as fatal or non-fatal ischaemic stroke, or fatal or non-fatal haemorrhagic stroke; and followed participants for at least one year. Two independent reviewers screened the titles, abstracts, and full texts of the initially identified studies to determine eligibility. Disagreements were resolved through consensus or consultation with a third author. To identify additional publications we searched the reference lists of all retrieved studies.
Data collection process and data items
Two independent reviewers used a predesigned data abstraction form to extract relevant information from the selected studies. The form included questions on study size, study design, baseline population (general populations or those at high risk of cardiovascular disease), geographical location, year of baseline survey, age range of participants at baseline, duration of follow-up, reported degree of adjustment for potential confounders (defined as “+” when relative risks were adjusted for age and sex, “++” after further adjustment for conventional vascular risk factors (for example, smoking status, history of diabetes, and hypertension), and “+++” when further adjusted for other additional variables (for example, social class or total energy intake), type and numbers of cerebrovascular disease outcomes, and reported relative risks for each outcome. Where appropriate we extracted information on type of fish (white or fatty), amount consumed daily, dietary assessment tool (dietary questionnaire, defined as food frequency or diet history questionnaires; diet records, defined as all open ended instruments such as 24 hour recall and food diaries), blinding status, and type and composition of supplement or placebo. In the case of multiple publications, we abstracted the most up to date or most comprehensive information.
Quality evaluation assessment
Two independent investigators evaluated the quality of the included studies by using a modified scoring system based on MOOSE, QUATSO, and STROBE guidelines.25 26 27 This allowed a total score from 0 to 6 points (6 representing the highest quality), with 1 point allocated if a study provided a study rationale; used appropriate selection criteria; employed a validated questionnaire (dietary studies) or assay method (blood based studies), or involved adequate blinding (randomised controlled trials); collected outcomes not solely based on self report; reported findings adjusted for age, sex, body mass index, and smoking status; and additionally adjusted for other covariates, such as social status and other nutrients.
Statistical analysis
To limit potential biases, the analyses involved only comparisons within studies (participants being directly compared only within each cohort). To enable a consistent approach to analyses, where appropriate, we transformed risk estimates from each prospective cohort study to enable comparisons between the top and bottom third of the population’s baseline distribution of exposure values, using methods previously described.28 Briefly, we transformed log risk estimates assuming a normal distribution, with the comparison between top and bottom thirds being equivalent to 2.18 times the log risk ratio for a 1 standard deviation increase (or equivalently as 2.18/2.54 times the log risk ratio for a comparison of extreme quarters). We calculated standard errors of the log risk ratios using published confidence limits and then standardised them in the same way. Hazard and odds ratios were assumed to approximate the same measure of relative risks. Using a random effects model that included between study heterogeneity, we calculated summary relative risks by pooling the study specific estimates. Where studies reported relative risks with differing degrees of adjustment for other risk factors, we used the maximum adjusted estimate. Dose-response meta-analysis of fish consumption and cerebrovascular risk was done using methods previously reported,24 29 which facilitated the calculation of a pooled relative risk across studies with a common unit of comparison within studies (the relative risk associated with a two incremental serving a week in this review), assuming a linear dose-response relation. Assessments for standardised categories of fish consumption (2-4 times a week and ≥5 times a week) and cerebrovascular risk compared with a reference category (≤1 a week) were based on combining comparable relative risk estimates across studies using random effects meta-analyses.22 As reference groups of the included studies varied (that is, from 0 to ≤1 times/week), in this analysis, we defined “≤1/week” as the reference category assuming that all reported reference exposures represent an equally low level of intake. Consistency of findings across studies was assessed by standard χ2 tests and the I2 statistic.30 Heterogeneity was quantified by comparing results from studies grouped according to prespecified study level characteristics using metaregression. Evidence of publication bias was assessed using funnel plots and the Egger test.31 All statistical tests were two sided and used a significance level of P<0.05. Analyses were done using Stata release 11.
Results
The search strategy identified 4343 unique citations. After the initial screening, based on titles and abstracts, 447 articles remained for further evaluation. Following detailed assessments, 402 articles were excluded (fig 1⇓). Overall, 45 articles based on 38 unique studies met the inclusion criteria and were included in the meta-analysis (see supplementary file). All relevant studies identified were published in the English language. In aggregate, the included studies comprised 794 000 unique participants and 34 817 incident cerebrovascular disease outcomes from 15 countries (table⇓ and supplementary tables).
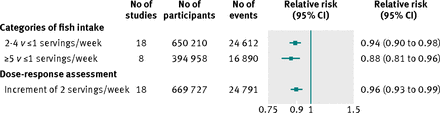
Fish consumption and cerebrovascular risk
Information on fish consumption and cerebrovascular disease were available in 21 prospective cohort studies, totalling 675 048 participants and 25 320 incident cerebrovascular events (see supplementary table 1). All 21 studies were based on general populations. Seven involved participants from Europe, seven from North America, and seven from the Asia-Pacific region. Six studies included only men, three only women, and the rest both sexes. The relative risk of cerebrovascular disease for standardised categories of fish intake, typically adjusted for several conventional risk factors, for 2-4 versus ≤1 servings a week was 0.94 (95% confidence interval 0.90 to 0.98) and for ≥5 versus ≤1 servings a week was 0.88 (0.81 to 0.96) (based on 18 and eight studies, respectively; fig 2⇓ and supplementary figs 1 and 2). There was no evidence of heterogeneity across studies (I2=20-22%, P>0.05 for both, see supplementary figs 1 and 2). In the dose-response meta-analysis (18 studies), an increment of two servings a week of any fish was associated with a 4% (95% confidence interval 1% to 7%) reduced risk of cerebrovascular disease (fig 2 and supplementary fig 3). For all 21 studies the relative risk when comparing participants in the highest with the lowest category of fish intake was 0.88 (0.84 to 0.93, see supplementary fig 4). In a subset of studies (62 799 participants) the corresponding relative risk for white fish types was 1.03 (0.90 to 1.19) and for fatty fish types was 0.84 (0.72 to 0.98, see supplementary fig 5).
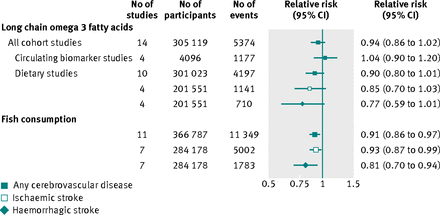
Comparison with evidence from long chain omega 3 fatty acid studies
Fourteen prospective studies reported on long chain omega 3 fatty acids in relation to risk of cerebrovascular disease, involving 305 119 participants and 5374 cerebrovascular outcomes recorded during an average follow-up ranging from four to 30 years (see supplementary table 2). Ten studies reported on intake of dietary long chain omega 3 fatty acid, whereas four were based on circulating blood compositions of omega 3. Five studies involved participants from Europe, four from North America, and five from the Asia-Pacific region. Seven studies concerned both sexes, five only men, and two only women. All cohorts included healthy populations at baseline. Fig 3⇓ and supplementary figs 6 and 7 present the relative risks for cerebrovascular disease for participants in the top compared with bottom third of baseline long chain omega 3 fatty acid and fish consumption. Relative risks for long chain omega 3 fatty acids measured as circulating biomarkers and self reported dietary exposures were 1.04 (95% confidence interval 0.90 to 1.20) and 0.90 (0.80 to 1.01), respectively. The corresponding relative risk in the top compared with bottom third of baseline fish consumption (11 studies; 366 787 participants) was 0.91 (0.86 to 0.97). Similar results were obtained for ischaemic and haemorrhagic stroke events (fig 3 and supplementary figs 8 and 9).
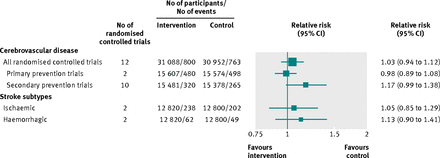
Effects of long chain omega 3 fatty acid supplements on cerebrovascular risk
Twelve randomised controlled trials totalling 62 040 participants reported on long chain omega 3 fatty acids supplementation and cerebrovascular disease (see supplementary table 3). No study assessed the effects of any dietary fish interventions. Most of these randomised controlled trials (nine out of 12) were done in Europe. Ten trials included participants with previous cardiovascular disease, whereas two concerned populations without any pre-existing cardiovascular disease at baseline. Participants in the intervention arm on average consumed 1.8 g of long chain omega 3 fatty acids daily (range 0.4-6.0 g/day), where an oil capsule was the principle form of supplementation. After an average follow-up of 3.0 (range 1.0-4.7 years) years, a total of 800 cerebrovascular events occurred among participants in the intervention group compared with 763 events in the control group, with a pooled relative risk of 1.03 (95% confidence interval 0.94 to 1.12; fig 4⇓). No evidence of heterogeneity was found among these randomised controlled trials (I2=3.5%, P=0.41, also see supplementary fig 10). The corresponding pooled relative risk for primary prevention trials (two studies) was 0.98 (0.89 to 1.08) and for secondary prevention trials (10 studies) was 1.17 (0.99 to 1.38, fig 4 and supplementary fig 10). The relative risks were broadly similar across baseline population and for cause specific cerebrovascular outcomes based on studies with available data (fig 4 and supplementary fig 10).
Stratified and sensitivity analyses
The overall associations observed in prospective studies for consumption of fish and long chain omega 3 fatty acids remained broadly consistent when these studies were grouped by several characteristics at study level. For instance, the overall relative risk of cerebrovascular disease when comparing the highest with the lowest category of fish intake was 0.87 (95% confidence interval 0.82 to 0.92) and for studies with higher and lower average fish consumption was 0.89 (0.78 to 1.01) (P for heterogeneity >0.05; see supplementary fig 11). Similarly, comparing studies with higher versus lower average consumption of long chain omega 3 fatty acids yielded relative risks of 0.93 (0.81 to 1.07) and 0.85 (0.72 to 1.01), respectively (P for heterogeneity >0.05, also see supplementary fig 12a). For consumption of both fish and omega 3 fatty acids in cohort studies, the magnitude of the relative risks of cerebrovascular disease for women seemed larger than those for men. In the randomised controlled trials there was also no clear evidence of heterogeneity according to several trial characteristics (for example, amount and type of intervention, blinding status, trial size, or duration of follow-up), except for some evidence that larger studies tended to yield more extreme relative risks (see supplementary fig 12b). In both observational and experimental studies, subgroup analyses indicated no geographical variation for the associations.
Analysis of publication bias
There was no evidence of publication bias across prospective cohort studies of fish or long chain omega 3 fatty acid consumption and the randomised controlled trials included in this review (P>0.05 in Egger’s asymmetry test for all, also see supplementary fig 13).
Discussion
This review found that higher fish consumption is moderately but significantly associated with a reduced risk of incident cerebrovascular disease. By contrast, dietary, circulating biomarkers and supplements of long chain omega 3 fatty acids were not significantly associated with risk of cerebrovascular disease. The combined estimates remained similar for cause specific cerebrovascular events. The associations for long chain omega 3 fatty acids were consistent across people with and without pre-existing cardiovascular disease—indicating a potential lack of benefit for either primary or secondary prevention of cerebrovascular disease. These findings therefore suggest that single nutrients may have limited effects on chronic disease outside of their original food sources.
The results observed in our review for fish consumption and cerebrovascular risk may have several alternative explanations. Firstly, it is possible that the potential benefit of fish consumption could in addition to long chain omega 3 fatty acids be attributed to a wider array of nutrients (and their interaction) that are abundant in fish. For example, fish are also a good source of vitamins D and B complex, which have been linked to inverse cerebrovascular risk.32 33 In addition, essential amino acids and trace elements in fish (for example, taurine,34 35 arginine,36 selenium,37 calcium,38 magnesium,39 40 potassium,41 and iodine42) may have potentially favourable vascular effects. Secondly, the positive impact of fish could be explained by a concomitant reduction in intake of foods detrimental to cerebrovascular health, such as red meat. Adjustment for confounding by other dietary factors is often inadequate and rarely accounts for such “protein substitution effect.”43 None the less, in a recent analysis based on two large cohorts in the United States, a 17% reduction in cerebrovascular risk was observed when red meat intake was replaced with fish.43 Thirdly, higher fish consumption may simply be an indicator of a healthier dietary pattern or higher socioeconomic status, which themselves are associated with conventional vascular determinants and outcomes.16 44 Although our subgroup analyses by levels of adjustment were consistent with the overall estimates, this may not fully address the potential issue of residual confounding, as individual characteristics such as baseline vascular drug use (antihypertensives, statins, aspirin, etc) or level of adherence (for instance for omega 3 supplements in randomised controlled trials) were generally unavailable in the studies included in the meta-analysis. The differences observed for the associations between white and fatty fish may be explained by the distinct ways in which the two foods are typically prepared. Studies that contributed to these analyses were exclusively based on the United Kingdom,17 where dietary questionnaires for the white fish items usually include battered and deep fried white fish; and in Sweden, where frying is the most common cooking method for white fish.15 This systematic difference in the preparation of white fish, in contrast with that of oily fish, could explain the null effect because potentially detrimental fats (such as trans fatty acids) are added during the cooking process.45
The discordant results observed for long chain omega 3 fatty acids compared with overall fish intake may also have various potential explanations. Firstly, food composition tables are often incomplete such that the long chain omega 3 fatty acid content of foods is unknown, which may lead to underestimation of the true intake of long chain omega 3 fatty acids. For example, only 65% of all foods in the US Department of Agriculture food composition database reportedly have a value for their omega 3 fatty acid content.46 Additionally, these databases may not account for all variations in the omega 3 fatty acid content of fish from different environments47 (for example, cooking methods, dietary patterns, or farm raised food versus wild sources).7 Secondly, self reported dietary questionnaires typically seek information on fish group rather than on specific fish types (for instance, oily fish versus salmon). Considering the substantial differences in quantity of omega 3 fatty acids between marine species,48 this inaccurate grouping of fish may lead to potential poor allocation of nutrient composition to food and overall misclassification of omega 3 fatty acid intake, which may attenuate the associations. Thirdly, although fish intake generally correlates highly with circulating blood omega 3 fatty acid levels, in the general Western population only 20-25% of the variation in such levels might be explained by fish consumption alone.49 This may be responsible, at least in part, for the inconsistent results between observational studies concerning fish and long chain omega 3 fatty acids. Fourthly, in our analyses circulating biomarkers of long chain omega 3 fatty acids were not associated with cerebrovascular disease. Although blood fatty acid levels are not affected by limitations in the dietary instruments or composition databases, these biomarkers only reflect exposures over the previous few weeks compared with a prolonged assessment that is more relevant to predict chronic disease risk.50 Finally, none of the randomised controlled trials were designed to investigate cerebrovascular disease as the primary outcome of interest. Therefore, competing risk events such as coronary heart disease may have altered the probability of cerebrovascular outcomes and impeded many subsequent events being considered thus limiting sufficiently powerful analyses. Furthermore, discrepant findings on omega 3 fatty acids in observational studies and randomised controlled trials may be partly explained by the inherent differences between baseline populations in the two designs—that is, generally healthy people compared with people with previous vascular disease or higher cardiovascular disease risk factors, respectively.
Comparison with previous studies
Findings of this updated meta-analysis generally concur and further extend the findings of previous reviews in several important ways. Our meta-analysis reinforces earlier results by including several recently published large scale prospective cohort studies. Our analyses on prospective dietary fish studies, for example, involved twice as many cerebrovascular events as the two previous reviews combined.22 24 Similarly, analyses on long chain omega 3 fatty acids based on data from observational studies and trials had about 10 times as many stroke events as the previous analysis.23 The current review also evaluates the potential effects of long chain omega 3 supplements on cerebrovascular risk by comparing observational evidence in the context of experimental data, systematically in a single investigation. Additionally, it comprehensively records a wide range of clinically relevant subgroups to carry out the most detailed exploration of potential heterogeneity than previously reported.
Implications of findings
Our findings may have several implications. They reinforce a potentially modest beneficial role of fish intake in the cause of cerebrovascular disease. Such an advantage was less evident for long chain omega 3 fatty acids in both observational studies and interventions targeting primary and secondary stroke prevention. Our findings are in line with current dietary guidelines (that is, to encourage fish consumption for all; and intake of fish oils, preferably from oily fish, to people with pre-existing or at high risk of coronary heart disease) and favour propositions that the future nutritional guidelines should be principally “food based.”51 They also underscore scientific gaps in the experimental evidence, specifically the lack of studies involving healthy populations and interventions targeting fish intake rather than using supplements, which may have different mechanistic effects. Additionally, adequately powered data from trials will be essential to investigate reliably the apparent higher risk of cerebrovascular disease observed in the secondary prevention trials of long chain omega 3 fatty acid supplements, and potential sex specific associations across observational studies.
Strengths and limitations of this review
The strengths and limitations of this review merit consideration. We included data from almost 800 000 participants from 15 countries. There was limited evidence of heterogeneity and publication bias across studies or within subgroups. In the absence of individual patient level data, we used standardised risk estimates to allow consistent comparisons and we examined several clinically relevant characteristics to reduce potential heterogeneity. However, the current review was limited by an overall lack of available data on the cause specific cerebrovascular outcomes. For example, only a few studies reported more than 1000 ischaemic stroke events, whereas most involved analyses primarily on composite cerebrovascular events. Furthermore, even in aggregate, fewer than 2000 cerebrovascular events were available in the randomised controlled trials and none of them were based on healthy populations.
Conclusions
Available observational data demonstrate moderate, inverse associations of fish and long chain omega 3 fatty acids consumption with risk of cerebrovascular events. However, there was no evidence for similar inverse associations with cerebrovascular disease for long chain omega 3 fatty acids measured as circulating biomarkers in observational studies, or supplements in primary and secondary prevention trials. The beneficial effect of fish intake on cerebrovascular risk might be mediated through a complex interplay among a wide range of nutrients commonly found in fish.
What is already known on this topic
Consumption of fish and long chain omega 3 fatty acids has been associated with a reduced risk of coronary heart disease and sudden cardiac death
However, observational and experimental evidence supporting a similar benefit for cerebrovascular disease remain conflicting
![]()
![]()

![]()
![]()
