文献:Luigi G et al.Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy.BMJ2013;347:f4587.
無作為化試験46件(被験者1万3532人)を対象に、ヘリコバクター・ピロリ除菌の順次療法の効果をシステマティックレビューなどで検討。順次療法の除菌率は84.3%で、7日および10日間3剤併用療法より優れていた(相対リスク1.21、1.11)。14日間3剤併用療法、ビスマス製剤含有療法、非含有療法には優越性を示さなかった。
Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy
BMJ 2013; 347 doi: http://dx.doi.org/10.1136/bmj.f4587 (Published 7 August 2013)
Cite this as: BMJ 2013;347:f4587
•
Clinical trials (epidemiology)
•
Infectious diseases
•
Internet
Article
Related content
Read responses (1)
Article metrics
Luigi Gatta, physician and gastroenterologist,
Nimish Vakil, clinical professor of medicine,
Dino Vaira, professor of internal medicine,
Carmelo Scarpignato, professor of pharmacology and therapeutics; associate professor of gastroenterology
Author Affiliations
1Gastroenterology and Endoscopy Unit, Versilia Hospital, Lido di Camaiore, Italy
2Department of Clinical and Experimental Medicine, University of Parma, Parma, Italy
3Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
4Department of Medical and Surgical Sciences, University of Bologna, Italy
Correspondence to: L Gatta, Clinical Pharmacology and Digestive Pathophysiology Unit, Department of Clinical and Experimental Medicine, University of Parma, 43125 Parma, Italy gattalg@gmail.com
Accepted 10 July 2013
Abstract
Objective To do a systematic review and meta-analysis of studies comparing sequential therapy for eradication of Helicobacter pylori with pre-existing and new therapies, thus providing a glimpse of eradication success worldwide.
Design Systematic review and meta-analysis.
Data sources Medline, Embase, and Cochrane Central Register of Controlled Trials up to May 2013; abstract books of major European, American, and Asian gastroenterological meetings.
Study selection Randomised controlled trials in previously untreated adults, in which sequential therapy was compared with a pre-existing or new therapy.
Results 46 randomised controlled trials were reviewed and analysed. 5666 patients were randomised to sequential therapy and 7866 to other (established and new) treatments. The overall eradication rate of sequential therapy was 84.3% (95% confidence interval 82.1% to 86.4%). Sequential therapy was superior to seven day triple therapy (relative risk 1.21, 95% confidence interval 1.17 to 1.25; I2=29.3%; number needed to treat 6 , 95% confidence interval 5% to 7%), marginally superior to 10 day triple therapy (1.11, 1.04 to 1.19; I2= 67.2%; NNT 10, 7 to 15), but not superior to 14 day triple therapy (1.00, 0.94 to 1.06; I2=54.3%), bismuth based therapy (1.01, 0.95 to 1.06; I2=21.1%), and non-bismuth based therapy (0.99, 0.94 to 1.05; I2=52.3%). Data on eradication according to pre-treatment antimicrobial susceptibility testing were available in eight studies, and sequential therapy was able to eradicate 72.8% (61.6% to 82.8%) of the strains resistant to clarithromycin.
Conclusions Eradication rates with pre-existing and new therapies for H pylori are suboptimal. Regional monitoring of resistance rates should help to guide treatment, and new agents for treatment need to be developed.
Introduction
Helicobacter pylori infection causes peptic ulcers, gastric mucosa associated lymphoid tissue lymphoma, and gastric cancer. Standard treatments for H pylori infection that have been endorsed by US as well as European scientific societies and by regulatory authorities rely on clarithromycin, metronidazole, or amoxicillin in conjunction with gastric acid inhibitors. The prevalence of resistance to clarithromycin and metronidazole has increased substantially in recent years, and a corresponding decrease has occurred in the eradication rate for H pylori infection, which has declined to unacceptable levels in most Western countries. A new treatment regimen that would achieve the eradication rates of 90% or greater seen at the advent of H pylori treatment is urgently needed. Such a regimen would need to have high efficacy against clarithromycin resistant and metronidazole resistant strains of H pylori, as these strains are increasingly encountered in routine clinical practice. As the response to eradication therapy is significantly related to the prevalence of primary resistance in the population, the choice of a treatment regimen should be based on the knowledge of the underlying prevalence of resistant strains in the community, which needs to be monitored.
Sequential therapy, a new regimen administering antimicrobials in a given sequence rather than all simultaneously, has generated worldwide interest. This kind of treatment is not actually new, as it uses established drugs, all approved for eradication of H pylori. However, the administration strategy is innovative. The sequential regimen is a simple dual therapy including a proton pump inhibitor plus amoxicillin 1 g (both twice daily) given for the first five days, followed by a triple therapy including a proton pump inhibitor, clarithromycin 500 mg, and a nitroimidazole antimicrobial (all twice daily) for the remaining five days. Initial studies of sequential therapy suggested that its superiority over standard triple therapy might be due to improved eradication of clarithromycin resistant strains.
Recently, several randomised controlled trials have compared sequential therapy with other established and new therapies. These provide a glimpse into eradication rates for H pylori in the countries where those studies were conducted. The aim of this study was to assess the efficacy of sequential therapy compared with other eradication regimens, by doing a systematic review and meta-analysis of randomised controlled trials.
Methods
Search strategy and study selection
This meta-analysis was developed according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement guidelines. We searched the medical literature by using Medline (1950 to May 2013), Embase (1980 to May 2013), and the Cochrane Central Register of Controlled Trials (May 2013). Randomised controlled trials examining the eradication rate of sequential therapy compared with other treatments were eligible for inclusion (box). We identified eligible studies with the terms “Helicobacter pylori”, “H. pylori”, “H pylori”, “Campylobacter pylori”, “C. pylori”, “C pylori”, “infection”, “dyspepsia”, “sequential”, “triple”, “concomitant”, “quadruple”, “treatment”, “therapy”, “omeprazole”, “lansoprazole”, “rabeprazole”, “pantoprazole”, “esomeprazole”, “bismuth”, “clarithromycin”, “metronidazole”, “tinidazole”, “amoxicillin”. We imposed no language restrictions. Two investigators (LG and NV) evaluated abstracts of the papers identified by the initial search for appropriateness, independently and in a blinded manner. Foreign language papers were translated where necessary. We also searched the abstract books from the British Society of Gastroenterology (2001-12), American Gastroenterological Association (2000-13), American College of Gastroenterology (2004-12), United European Gastroenterology Week (2000-12), European Helicobacter pylori Study Group (2000-12), and Asian Pacific Digestive Week (2003-12). We used bibliographies of all relevant studies identified to do a recursive search. In addition, we contacted authors to obtain unpublished data from their studies, whenever we deemed it necessary.
Eligibility criteria
Randomised controlled trials*
Patients aged ≥18 years
Patients never treated before for Helicobacter pylori infection
Patients without significant comorbidity (for example, renal failure, hepatic failure, cancer)
Helicobacter pylori infection diagnosed (before and after treatment) using at least one of histology, rapid urease test, 13/14C urea breath test, stool test, culture†
Randomised controlled trials comparing sequential treatment‡ with other eradication regimens
Eradication rate according to intention to treat analysis
Eradication assessed at least four weeks after end of treatment
*Articles and/or abstracts reporting only interim analysis of randomised controlled trials were not included
†Articles and/or abstracts not reporting test used to diagnose infection and/or to follow-up infection were not included
‡Sequential treatment defined as proton pump inhibitors twice daily + amoxicillin 1 g twice daily for five days followed by proton pump inhibitors twice daily + clarithromycin 500 mg twice daily + nitroimidazole derivatives twice daily for next five days
Outcome assessment
The primary outcome was the efficacy of sequential therapy compared with established and new therapies in eradicating H pylori infection. Secondary outcomes included safety and efficacy according to the antimicrobial resistance pattern, where reported.
Data extraction
Two investigators (LG and NV) assessed articles independently, using pre-designed data extraction forms. Disagreement between investigators was resolved by discussion with the other two investigators (DV and CS). Data on eradication were based on intention to treat analysis. In addition, the following clinical data were extracted for each trial: country of origin, type of publication (article, abstract), proton pump inhibitor used, use of tinidazole (versus other nitroimidazole derivatives), duration of comparative eradication treatment, and adverse event rate.
Evaluation of risk of bias
We assessed risk of bias as described in the Cochrane handbook, by evaluating the random sequence generation, concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. We considered randomised controlled trials as being at low risk of bias if all the domains except blinding of participants or personnel were properly assured. As the outcome (that is, eradication) was almost always assessed by objective means, we did not consider blinding to be crucial.
Statistical analysis
We assessed data for the primary outcome by using a random effects model, to give a conservative estimate of the 95% confidence intervals. Results were expressed as relative risk for success of H pylori eradication and as difference in eradication rates among patients assigned to sequential therapy versus other eradication regimens. We also used a random effects model to pool data for safety, and expressed them as relative risk for adverse events. We required at least three comparable study groups for every comparison for randomised controlled trials to be included in the meta-analysis. We also calculated prediction intervals at 95% confidence intervals for the primary outcome, as they might be considered a more appropriate future treatment summary.
We assessed heterogeneity between trials with the χ2 test for heterogeneity at a significance level of P<0.1. We also calculated the I2 statistic. Its value ranges from 0% to 100%, with 0% representing no observed heterogeneity and larger values indicating increasing heterogeneity. We chose a value below 25% to represent low levels of heterogeneity. When the degree of statistical heterogeneity was greater than this cut-off between trial results for the primary outcome, we investigated possible explanations by using subgroup analyses according to country of origin, use of tinidazole as the nitroimidazole derivative in the sequential treatment, type of publication (abstract versus article), proton pump inhibitor used, duration of comparative treatment (when applicable), and trials with a high risk and unclear risk of bias versus a low risk of bias. As exploratory analyses, they may explain some of the observed variability between trials. We used the Cochran Q statistic to compare the relative risks between studies in the analyses.
We used a random effects model to calculate eradication rates of regimens.We calculated proportions, their differences, and 95% confidence intervals by using the method recommended by Newcombe and Altman. We calculated the number needed to treat and 95% confidence intervals from the reciprocal of the risk difference of the meta-analysis. We used Stata version 10.1 to generate forest plots for primary and secondary outcomes with 95% confidence intervals, as well as funnel plots. We assessed funnel plots for evidence of asymmetry and possible publication bias or other small study effects, by using the Egger’s linear regression and regarding a two sided P value of 0.10 or less as significant.
Results
The search strategy we used identified 610 citations, of which we excluded 545 after examining the title and abstract. We retrieved and evaluated 65 articles in more detail. Of these, we excluded 19 for various reasons, leaving 46 randomised controlled trials that were eligible for inclusion, as shown in figure 1, 11 of which were abstracts. Fifteen studies included more than two arms. Three studies could not be included in the meta-analysis because our criteria required at least three comparable study groups for every comparison. Only four trials were at low risk of bias. The table shows detailed characteristics of the studies included in the systematic review and meta-analysis. Supplementary table A shows a complete evaluation of risk of bias.
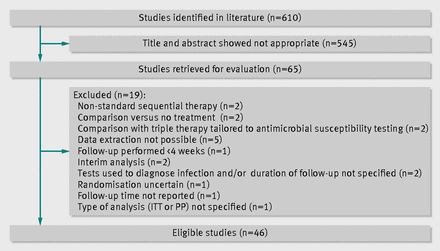
Fig 1 Flow diagram of systematic review. ITT=intention to treat; PP=per protocol.
Characteristics of studies included in systematic review and meta-analysis
Meta-analysis
Sequential therapy versus triple therapy lasting seven days
Twenty two studies compared sequential therapy with a triple therapy regimen lasting seven days. No trial was at low risk of bias, and two trials did not report full data on the proton pump inhibitor used. As shown in figure 2, the pooled relative risk was 1.21 (95% confidence interval 1.17 to 1.25), favouring sequential treatment, the number needed to treat was 6 (95% confidence interval 5 to 7), and the 95% prediction intervals ranged from 1.10 to 1.33. We found evidence of heterogeneity (I2=29.3%; P=0.098), without funnel plot asymmetry (Egger’s test coefficient −0.69, 90% confidence interval −1.71 to 0.33; P=0.257).
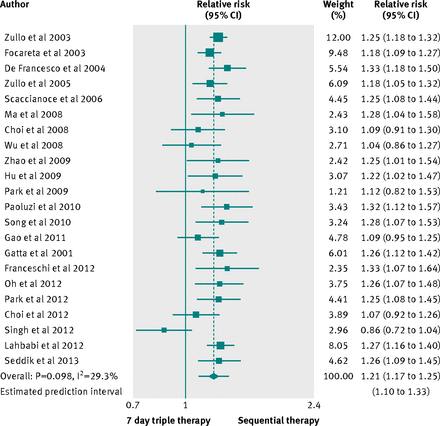
Fig 2 Forest plot of sequential therapy versus seven day triple therapy
In all, 2449 patients were treated with the sequential therapy compared with 2566 patients treated with triple therapy lasting seven days, and the eradication rate reported was 86.5% (95% confidence interval 82.9% to 89.7%) for the sequential therapy and 71.5% (68.4% to 74.5%) for the triple therapy. The difference in eradication rates was 15% (13% to 18%) favouring sequential treatment, and the 95% prediction intervals ranged from 9% to 22% (supplementary figure A), with evidence of heterogeneity (I2=34.0%; P=0.061).
Because of the heterogeneity, we did subgroup analyses according to country of origin, use of tinidazole in the sequential therapy, type of publication, and proton pump inhibitor used; we did not evaluate risk of bias, as all trials were at high or unclear risk of bias (supplementary table B). We found a slightly statistically significant effect in favour of sequential therapy in trials conducted in China, Italy, Korea, and Morocco.
One of these studies compared sequential therapy with both proton pump inhibitor-amoxicillin-clarithromycin and proton pump inhibitor-amoxicillin-metronidazole. The eradication rate of sequential therapy was 24.0% (13.6% to 33.5%) higher than proton pump inhibitor-amoxicillin-clarithromycin and 15.9% (7.1% to 25.1%) higher than proton pump inhibitor-amoxicillin-metronidazole. One trial also compared sequential therapy with a modified triple therapy (and for this reason we did not include this arm in the meta-analysis) consisting of proton pump inhibitor twice daily, clarithromycin 500 mg twice daily, and amoxicillin 1000 mg three times daily for seven days. No significant difference in eradication rate was observed between the two treatments (P=0.750).
Data on adverse events were available in 18 trials.
![]()
![]()

![]()
![]()
