文献:Surén P et al.Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children.JAMA. 2013;309(6):570-577.
ノルウェー母子コホート研究(MoBa)に参加した小児8万5176人を対象に、妊娠前後の母親の葉酸摂取と子の自閉症スペクトラム障害リスクの関連を検討。自閉性障害の発生率は葉酸摂取群0.10%、非摂取群0.21%だった(調整後オッズ比0.61)。アスペルガー症候群または特定不能の広汎性発達障害との関連は特定できなかった。
Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in ChildrenFREE
Pål Surén, MD, MPH; Christine Roth, MSc; Michaeline Bresnahan, PhD; Margaretha Haugen, PhD; Mady Hornig, MD; Deborah Hirtz, MD; Kari Kveim Lie, MD; W. Ian Lipkin, MD; Per Magnus, MD, PhD; Ted Reichborn-Kjennerud, MD, PhD; Synnve Schjølberg, MSc; George Davey Smith, MD, DSc; Anne-Siri Øyen, PhD; Ezra Susser, MD, DrPH; Camilla Stoltenberg, MD, PhD
[+-] Author Affiliations
Author Affiliations: Norwegian Institute of Public Health, Oslo (Drs Surén, Haugen, Lie, Magnus, Reichborn-Kjennerud, Øyen, and Stoltenberg and Mss Roth and Schjølberg); Centre for Paediatric Epidemiology and Biostatistics, UCL Institute of Child Health, London, United Kingdom (Dr Surén); Mailman School of Public Health, Columbia University, New York, New York (Drs Bresnahan, Hornig, Lipkin, and Susser and Ms Roth); New York State Psychiatric Institute, New York (Drs Bresnahan and Susser); National Institute of Neurological Disorders and Stroke, Bethesda, Maryland (Dr Hirtz); Institute of Psychiatry, University of Oslo, Oslo (Dr Reichborn-Kjennerud); MRC Centre for Causal Analysis in Translational Epidemiology, University of Bristol, Bristol, United Kingdom (Dr Davey Smith); Nic Waals Institute, Lovisenberg Hospital, Oslo (Dr Øyen); and Department of Public Health and Primary Health Care, University of Bergen, Bergen, Norway (Dr Stoltenberg).
JAMA. 2013;309(6):570-577. doi:10.1001/jama.2012.155925.
Text Size: AA A
Published online
Article
Figures
Tables
References
ABSTRACT
ABSTRACT | METHODS | RESULTS | COMMENT | AUTHOR INFORMATION | REFERENCES
Importance Prenatal folic acid supplements reduce the risk of neural tube defects in children, but it has not been determined whether they protect against other neurodevelopmental disorders.
Objective To examine the association between maternal use of prenatal folic acid supplements and subsequent risk of autism spectrum disorders (ASDs) (autistic disorder, Asperger syndrome, pervasive developmental disorder–not otherwise specified [PDD-NOS]) in children.
Design, Setting, and Patients The study sample of 85 176 children was derived from the population-based, prospective Norwegian Mother and Child Cohort Study (MoBa). The children were born in 2002-2008; by the end of follow-up on March 31, 2012, the age range was 3.3 through 10.2 years (mean, 6.4 years). The exposure of primary interest was use of folic acid from 4 weeks before to 8 weeks after the start of pregnancy, defined as the first day of the last menstrual period before conception. Relative risks of ASDs were estimated by odds ratios (ORs) with 95% CIs in a logistic regression analysis. Analyses were adjusted for maternal education level, year of birth, and parity.
Main Outcome Measure Specialist-confirmed diagnosis of ASDs.
Results At the end of follow-up, 270 children in the study sample had been diagnosed with ASDs: 114 with autistic disorder, 56 with Asperger syndrome, and 100 with PDD-NOS. In children whose mothers took folic acid, 0.10% (64/61 042) had autistic disorder, compared with 0.21% (50/24 134) in those unexposed to folic acid. The adjusted OR for autistic disorder in children of folic acid users was 0.61 (95% CI, 0.41-0.90). No association was found with Asperger syndrome or PDD-NOS, but power was limited. Similar analyses for prenatal fish oil supplements showed no such association with autistic disorder, even though fish oil use was associated with the same maternal characteristics as folic acid use.
Conclusions and Relevance Use of prenatal folic acid supplements around the time of conception was associated with a lower risk of autistic disorder in the MoBa cohort. Although these findings cannot establish causality, they do support prenatal folic acid supplementation.
Figures in this Article
Supplementation with folic acid around the time of conception reduces the risk of neural tube defects in children. This protective effect has led to mandatory fortification of flour with folic acid in several countries, and it is generally recommended that women planning to become pregnant take a daily supplement of folic acid starting 1 month before conception.
There also is evidence that maternal folic acid supplementation during pregnancy may be associated with reduced risk of other neurodevelopmental disorders in children. A recent study of 38 954 children in the Norwegian Mother and Child Cohort Study (MoBa) found that maternal intake of folic acid supplements from 4 weeks before to 8 weeks after the start of pregnancy was associated with a lower risk of severe language delay at age 3 years. A case-control study from California of autism spectrum disorders (ASDs) showed that maternal intake of folic acid and prenatal vitamins during the 3 months prior to pregnancy and the first month of pregnancy was associated with a lower risk of ASDs in the offspring, and complementary genetic analyses indicated that the association was modified by gene variants that determine the ability to utilize available folate.
Although ethical considerations preclude placebo-controlled randomized trials that eliminate folic acid, observational studies of mothers who do and do not use supplements may be informative. We used the MoBa cohort to investigate the association between the use of maternal folic acid supplements before and in early pregnancy and the subsequent risk of ASDs (autistic disorder, Asperger syndrome, pervasive developmental disorder–not otherwise specified [PDD-NOS]) in the offspring.
METHODS
ABSTRACT | METHODS | RESULTS | COMMENT | AUTHOR INFORMATION | REFERENCES
Study Population
The MoBa cohort is nationwide and includes 109 000 children born from 1999 to 2009. Mothers were recruited at ultrasound examinations around week 18 of gestation. Cases of ASD in the cohort are identified by a substudy of autism, the Autism Birth Cohort (ABC) study. The analyses in this study reflect data collected and processed by March 31, 2012. Participation in MoBa and the ABC study is based on written informed consent from the mother. Both studies were approved by the regional committee of medical research ethics for Southeastern Norway.
Measures of ASD
Cases of ASD are identified through (1) questionnaire screening of mothers at offspring ages 36 months, 5 years, and 7 years, (2) professional and parental referrals of children suspected of having ASD, and (3) linkages to the Norwegian Patient Registry. Referrals are elicited through annual newsletters to MoBa participants and information on the Norwegian Institute of Public Health website. The Norwegian Patient Registry collects data on diagnoses from all hospitals and outpatient clinics in Norway beginning in the year 2008, thereby capturing data for all children diagnosed with ASD by Norwegian health services.
When a child with ASD or potential ASD is detected through any of the mechanisms described above, he or she is invited to participate in a clinical assessment that includes the research-standard instruments for diagnosis of ASD, the Autism Diagnostic Interview–Revised and the Autism Diagnostic Observation Schedule, which have proven high reliability and validity in making diagnoses of ASD in children. Assessments are conducted without knowledge of previous questionnaire responses. Diagnostic conclusions are best-estimate clinical diagnoses derived from test and interview results and from information collected from parents and teachers. Diagnoses are based on Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria, and the case definition includes codes 299.00 (Autistic Disorder), 299.80 (Asperger Syndrome), and 299.80 (Pervasive Developmental Disorder–Not Otherwise Specified).
The registry contains International Statistical Classification of Diseases, 10th Revision codes determined by Norwegian specialist health services, and the ASD case definition of the ABC study includes codes F84.0 (Childhood Autism), F84.1 (Atypical Autism), F84.5 (Asperger Syndrome), F84.8 (Other Pervasive Developmental Disorder), and F84.9 (Pervasive Developmental Disorder, Unspecified). In this article, we have used the terms autistic disorder for code F84.0 and PDD-NOS for codes F84.1, F84.8, and F84.9.
Measures of Folic Acid Use and Dietary Folate Intake
Since 1998, the Norwegian Directorate of Health has recommended that all women attempting to become pregnant should take one 400-μg folic acid supplement per day from 1 month before conception through the first trimester. Folic acid supplements are available over the counter in Norway. Multivitamin supplements containing folic acid are also available, but at the time participants were recruited to MoBa, all such supplements contained less than 400 μg of folic acid.
In MoBa, detailed information about the mothers' supplement intake before conception and in early pregnancy was obtained through questionnaire report at week 18 of gestation. No foods were fortified with folic acid at the time when participants were recruited; synthetic supplements thus represented the only source of folate apart from the ordinary diet for the pregnant women. The women were asked to record their intake of vitamins, minerals, and other supplements according to the ingredient lists on the supplement containers, within 4-week intervals from before the start of pregnancy. They were not asked to specify the exact amounts, so if folic acid was only taken as part of a multivitamin supplement, the daily dose would be lower than 400 μg.
Additional information about supplement use and dietary intake in mid pregnancy was obtained through a food frequency questionnaire completed in week 22. In this questionnaire, women were asked to write the name of supplements they were currently taking (in week 22), and exact amounts of vitamins and minerals were calculated on the basis of this information. The food frequency questionnaire has been described in a previous article and validated through blood samples and 4-day food records from a subsample of the cohort.
Measures of Timing
For our primary analyses, we examined an interval from 4 weeks before to 8 weeks after the start of pregnancy. The start of pregnancy was defined as the first day of the last menstrual period before conception, in keeping with the standard definition used in the follow-up of pregnant women in Norway. Children of mothers who used folic acid supplements during the entire or parts of the exposure interval were compared with children whose mothers did not use folic acid supplements during the interval. The exposure interval was chosen on the basis of an a priori hypothesis that the effect of folic acid on the development of the central nervous system is most prominent in this period, and it also corresponds to the interval used in the previous study of language delay. The interval covers or precedes events of critical importance to the fetal brain, such as the closure of the neural tube 28 days after conception (gestational week 6) and the embryonic period that includes development of the basic brain structures 15 to 56 days after conception (gestational weeks 5-10).
Potential Confounders
We explored a number of factors that might influence a potential association between supplement use and ASD risk: parental education, parental age, whether the pregnancy was planned, maternal smoking during pregnancy, maternal body mass index (calculated as weight in kilograms divided by height in meters squared), weight gain at weeks 18 and 30, parity, and year of birth.
Statistical Analyses
Analyses were performed using SPSS version 19.0 (SPSS Inc). Odds ratios (ORs) with 95% CIs for the association between folic acid use and risk of each ASD were estimated from logistic regression models. The adjusted models included adjustment for year of birth, maternal education level, and parity, because these were the only covariates that had any influence on the OR estimates.
We estimated the power of these analyses for autistic disorder, which was the ASD subtype with the highest number of diagnosed cases in the study sample. The power calculations were based on the observed distributions of the outcome and the exposure, ie, an overall prevalence of 0.13% for autistic disorder and a proportion of 68% of the study sample exposed to folic acid within the exposure interval. The type I error probability was set at α = .05 (2-sided). Under these conditions we had a power of 93% to detect an OR of 0.50, 73% to detect an OR of 0.60, 45% to detect an OR of 0.70, and 18% to detect an OR of 0.80.
To assess the possibility of residual confounding, we explored whether maternal illness and medication use during pregnancy had any effect on association. Information about maternal illness and medication use was obtained from the questionnaire completed in week 18 and from the Medical Birth Registry. We adjusted the logistic regression models for the presence of anxiety, depression, epilepsy, preeclampsia, and diabetes during pregnancy (separately for each disorder). We also made separate adjustments for use of medications for anxiety, depression, and epilepsy and for the use of hormone treatment and in vitro fertilization to become pregnant.
We conducted a secondary analysis of the association between maternal use of fish oil supplements and the risk of ASD, to investigate whether the associations were specific to folic acid or similar across different types of supplements. If they were similar, the associations would more likely be attributable to health-conscious maternal behaviors in general and not the supplements per se. We also examined the association between folic acid use in week 22 of pregnancy and subsequent risk of ASD, to evaluate whether any associations, if present, were similar in early pregnancy and mid pregnancy.
The study did not have sufficient power for subgroup analyses, but we performed some exploratory analyses for the autistic disorder subtype to look for clues to interactions that could be tested in future studies. We explored (1) the timing of initiation of folic acid supplementation; (2) maternal use of other vitamins, minerals, or both from 4 weeks before to 8 weeks after the start of pregnancy; (3) maternal total daily intake of folate in week 22 (diet and supplements combined, adjusted for dietary folate equivalents); (4) stratification of autistic disorder cases by language level at 36 months; and (5) stratification of the study sample by year of birth (2002-2004 vs 2005-2008).
RESULTS
ABSTRACT | METHODS | RESULTS | COMMENT | AUTHOR INFORMATION | REFERENCES
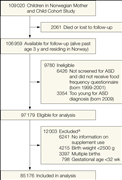
The derivation of the study sample is shown in Figure 1. A total of 97 179 cohort participants were eligible for the analyses. To isolate folic acid exposure from other exposure reported to increase the risk of ASD, we excluded children with gestational age less than 32 weeks at birth, children with birth weight less than 2500 g, and multiple births. We also excluded children for whom we did not have data on maternal supplement use before conception and in early pregnancy as well as children whose mothers reported supplement use but had not specified the type and duration. In total, 12 003 children were excluded, for 1 or more reasons. The final study sample included 85 176 children. At the end of follow-up, the age range was 3.3 through 10.2 years (mean, 6.4 years).
Grahic Jump Location
Place holder to copy figure label and caption
Figure 1. Derivation of the Study Sample
Approximately 283 000 children were invited to participate in the Norwegian Mother and Child Cohort Study; of these, approximately 174 000 declined to participate. ASD indicates autism spectrum disorder. aNumbers sum to more than 12 003 because some children met more than 1 exclusion criterion.

A total of 270 children (0.32%) in the study sample have been diagnosed with ASDs: 114 (0.13%) with autistic disorder, 56 (0.07%) with Asperger syndrome, and 100 (0.12%) with PDD-NOS. The distribution of ASD cases by year of birth is shown in eTable 1. Of the ASD cases, 135 (50.0%) had been clinically assessed through the ABC study. The remaining 135 had specialist-confirmed diagnoses of ASD recorded in the Norwegian Patient Registry. Registry diagnoses had a high validity for ASD as a whole: of the 39 children assessed in the ABC study after being detected through the registry, 38 were found to meet DSM-IV criteria for ASD, generating a positive predictive value (PPV) of 97% (95% CI, 87%-100%). Estimates of PPV are lower for the individual ASD subtype diagnoses: 80% (12/15 [95% CI, 52%-96%]) for autistic disorder, 38% (5/13 [95% CI, 14%-68%]) for Asperger syndrome, and 73% (8/11 [95% CI, 39%-94%]) for PDD-NOS. Estimates of PPV for the subtype diagnoses are preliminary, because the number of cases in each group is still low.
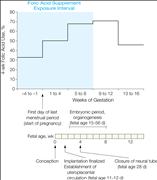
The proportions of mothers reporting folic acid use are shown in Figure 2. In the first interval (weeks 4 to 1 before the start of pregnancy), 32.9% of mothers took folic acid. The proportion increased to 70.7% in weeks 9 through 12 and then reverted to 45.8% in weeks 13 through 16. The distribution of folic acid use across categories of parent and child characteristics is shown in Table 1. Women who used folic acid within the exposure interval (4 weeks before to 8 weeks after the start of pregnancy) were more likely to have college- or university-level education, to have planned the pregnancy, to be nonsmokers, to have prepregnancy body mass index below 25, and to be first-time mothers. Folic acid use increased substantially by year of birth, from 43.2% in 2002 to 83.7% in 2008.
Grahic Jump Location
Place holder to copy figure label and caption
Figure 2. Folic Acid Supplement Use by Pregnancy Interval


Women who took folic acid in early pregnancy had a higher response rate to the screening questionnaire completed when the children were aged 36 months. For the study sample overall, the response rate was 62% in folic acid users and 55% in nonusers. For children born in 2005-2008, ie, the youngest children, the difference was somewhat larger, with a response rate of 61% in folic acid users and 50% in nonusers. Consequently, children with ASDs born to women who used folic acid may have had a higher probability of being diagnosed at an early age.
Results of the logistic regression analysis for autistic disorder are reported in Table 2. There was an inverse association between folic acid use and subsequent risk of autistic disorder. Autistic disorder was present in 0.10% (64/61 042) of children whose mothers took folic acid, compared with 0.21% (50/24 134) in children whose mothers did not take folic acid. The adjusted OR of autistic disorder was 0.61 (95% CI, 0.41-0.90) in children of folic acid users. Adjustment for maternal illness and medication use did not affect the OR (eTable 2).
Table Grahic Jump LocationTable 2. Risk of Autistic Disorder According to Maternal Folic Acid Use

The use of fish oil supplements followed patterns similar to those for folic acid use in the study sample: it was associated with the same parental characteristics (eTable 3), it increased throughout the period of recruitment to the cohort (eTable 3), and it increased from before pregnancy through the first trimester (eFigure). Despite these similarities, there was no association between use of fish oil supplements and risk of autistic disorder, as shown in Table 3. The adjusted OR of autistic disorder was 1.29 (95% CI, 0.88-1.89) in children of mothers who used fish oil supplements.
Table Grahic Jump LocationTable 3. Risk of Autistic Disorder According to Fish Oil Use in Weeks −4 to 8 and Folic Acid Use in Week 22

The inverse association found for folic acid use in early pregnancy was absent for folic acid use in mid pregnancy: the adjusted OR for autistic disorder was 0.96 (95% CI, 0.60-1.55) for mothers taking 400 μg or more per day in week 22 and 1.02 (95% CI, 0.62-1.67) for those taking less than 400 μg per day at that time (Table 3).
For Asperger syndrome and PDD-NOS, we restricted the analyses to birth years with a cumulative incidence of 0.08% or higher (higher than the lowest level observed for autistic disorder): 2002-2004 for Asperger syndrome (n = 30 117, including 48 cases) and 2002-2006 for PDD-NOS (n = 59 152, including 91 cases). Our power to detect an OR of magnitude similar to that found for autistic disorder (OR, 0.61) was limited: 36% for Asperger syndrome and 61% for PDD-NOS. For Asperger syndrome, the proportion of diagnosed cases was 0.12% (21/17 218) in children of folic acid users and 0.21% (27/12 899) in children of nonusers, generating an adjusted OR of 0.65 (95% CI, 0.36-1.16). For PDD-NOS, the proportion was 0.15% (58/39 543) in children of folic acid users and 0.17% (33/19 649) in children of nonusers, generating an adjusted OR of 1.04 (95% CI, 0.66-1.63).
Results of exploratory analyses are reported in Table 4. These results should be cautiously interpreted, because none of the exploratory analyses had a statistical power of more than 50% to detect an OR of 0.60. There did not seem to be a strong gradient in risk by timing of initiation of folic acid use within the primary exposure interval. The use of other vitamins and minerals in addition to folic acid did not appear to affect risk of autistic disorder. The analyses based on the food frequency questionnaire data from week 22 did not reveal any apparent association between maternal total daily folate intake in week 22 (diet and supplements combined) and subsequent risk of autistic disorder in children. The analysis in which cases were stratified according to language level suggested that the inverse association may be strong in children with severe language delay and weak in those with moderate or no delay. The analysis stratified by year of birth suggested that the inverse association may be stronger in the older children (born in 2002-2004) than in the younger children (born in 2005-2008).
Table Grahic Jump LocationTable 4. Exploratory Analyses
COMMENT
ABSTRACT | METHODS | RESULTS | COMMENT | AUTHOR INFORMATION | REFERENCES
This study found that maternal use of supplemental folic acid from 4 weeks before to 8 weeks after the start of pregnancy was associated with a lower risk of autistic disorder—the most severe form of ASD—in children.
Use of folic acid supplements was associated with higher socioeconomic status and more health-conscious maternal behavior patterns in the study sample. We cannot exclude the possibility that some portion of the inverse association represents residual, unmeasured confounding. However, if residual confounding was substantial, we would have expected to find a lowering of risk associated with fish oil supplement use as well, because the use of fish oil was associated with the same parental characteristics in the study sample. No such lowering of risk was observed. We also would have expected the inverse association between folic acid use and autistic disorder risk to persist in mid pregnancy (week 22), which it did not. There was no evidence of residual confounding in analyses adjusting for maternal illness and medication, which might reflect the fact that the pregnant women in the cohort were generally healthy and had low proportions of medication use during pregnancy. We did not have data on more rare psychiatric disorders, but we believe that such disorders are unlikely to have had any significant influence.
Our findings indicate that the inverse association may be largely driven by the children with autistic disorder and severe language delay at 36 months, who were presumably the more severely affected children. It is also worth noting that the OR estimate for those with missing data on language level (nonresponders to the screening questionnaire) was similar to that for those with severe language delay. This suggests that mothers with severely affected children may have had lower response rates and that an ascertainment bias may have been present. The possibility of such bias, combined with the small numbers in each stratum, warrants caution in the interpretation of these findings.
The participation rate among women invited to participate in MoBa was 38.5%, and the cohort is not fully representative of the Norwegian population. Comparisons with nationwide registry data have demonstrated that the mothers in the cohort were more likely to be first-time mothers, less likely to be single mothers, and had higher levels of education, higher mean age, and lower levels of smoking than other pregnant women.
We tested the generalizability of our findings by replicating the analyses in a nationwide data file containing data from the Medical Birth Registry of Norway, the Norwegian Patient Registry, and Statistics Norway. We included children born in 1999-2007 and applied the same inclusion criteria as for the MoBa-based analyses. The replication sample included 473 095 children, of whom 822 (0.17%) had diagnoses of autistic disorder recorded by Norwegian specialist health services. Folic acid use is substantially underreported in the Medical Birth Registry; our comparisons with the MoBa questionnaire data found that half of the mothers reported in the registry to be nonusers of folic acid actually had reported folic acid use in the questionnaires. This underreporting is a major limitation and will bias association measures toward the null. Despite this, we did find a significant inverse association in the nationwide sample: mothers with reported folic acid use had an adjusted OR of 0.83 (95% CI, 0.71-0.97) for autistic disorder in their children. When the MoBa participants were analyzed using the registry-based folic acid variable, the adjusted OR was 0.75 (95% CI, 0.46-0.96). The similarity between MoBa participants and nonparticipants suggest that our MoBa-based analyses have not been significantly affected by selection bias.
The strengths of the study were the cohort design, large sample size, and prospective data collection as well as the combination of screening, referrals, and registry linkage for detection of ASD cases. The richness of the exposure data allowed for differentiations between different supplements and between the various stages of pregnancy. Our ability to compare the study sample with a nationwide sample was also an advantage.
The main limitation was the incomplete ascertainment of ASD cases in the cohort. The prevalence of diagnosed ASD was lower than what has been reported in the United Kingdom and the United States, although that discrepancy is not merely attributable to underascertainment, because the nationwide ASD prevalence is also lower in Norway. For the country as a whole, the prevalence is estimated to be 0.8% in 12-year-olds,24 which is not very different from the 0.66% prevalence observed for children born in 2002 and 2003 in the MoBa cohort (eTable 1). Underascertainment was less of a problem for autistic disorder than for the other ASD subtypes, but it was still reassuring that the inverse association for autistic disorder was stronger in the older children (born in 2002-2004), for whom case ascertainment was closer to completion. The relative weakness of the inverse association among the younger children (born in 2005-2008) may have resulted from the fact that mothers who took prenatal folic acid supplements had higher questionnaire response rates, causing the ASD cases among their children to be identified earlier. As mentioned previously, there also was the possibility of ascertainment bias arising from lower response rates among parents of the children more severely affected with autistic disorder. If such bias were present, the OR estimate for the younger children would be biased toward the null.
Another limitation of the study was the reliance on subtype diagnoses of ASD. These have not been found to have high reliability across assessment sites in studies in the United States and may be removed altogether from the upcoming DSM-V classification system.25 Our own validation of registry diagnoses indicated that subtype diagnoses were less reliable than for ASD as a whole, but there was still a high level of agreement (PPV, 80%) for autistic disorder diagnoses, which was the outcome of primary interest.
Our main finding was that maternal use of folic acid supplements around the time of conception was associated with a lower risk of autistic disorder. This finding does not establish a causal relation between folic acid use and autistic disorder but provides a rationale for replicating the analyses in other study samples and further investigating genetic factors and other biological mechanisms that may explain the inverse association.
![]()
![]()